Under Which Conditions Will Gases Best Dissolve in Liquids
While it is in general true for gases dissolved in water gases dissolved in organic solvents tend to become more soluble with increasing temperature. An increase in temperature leads to an increase in kinetic energy.

Gas Solubility And Temperature Introduction To Chemistry
The amount of gas that can be dissolved in water depends on the temperature of the water.
. Gas is soluble in more liquids than just water so there isnt any specific requirement for the liquid to be moist in order for the gas to become dissolved in the liquid. While exceptions may occur at very high pressures the solubility of a gas in a liquid generally rises as the pressure of that gas increases. The magnitude of the solubility can be enormous or minuscule depending upon the gas and the.
Dissociation is a common way for things to be dissolved in liquids. This creates a solution a mixture where the gas is the solute the minor component and the other material. The dissolving of a gas in water depends on the interaction between the molecules of the gas and the water molecules.
A gas dissolves in a liquid most rapidly when under _______ pressure. Under which conditions will gases best dissolve in liquids. Higher kinetic energy causes the gas molecules to break their intermolecular bonds and escape from solution.
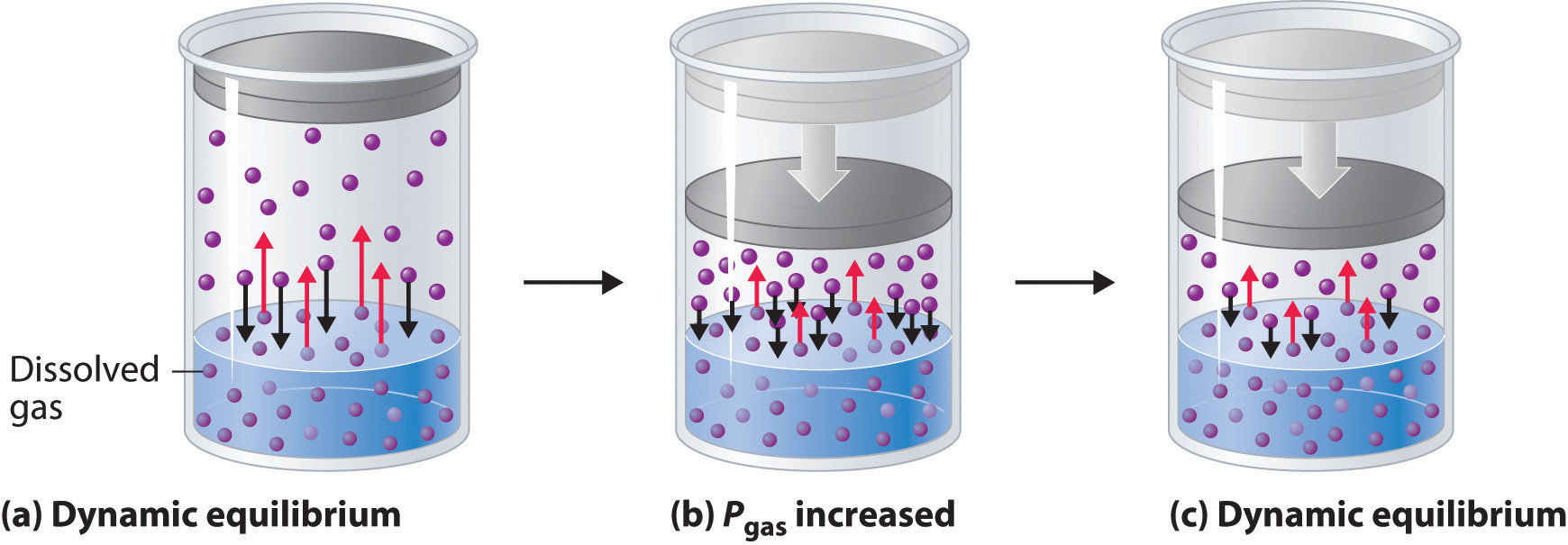
The gas molecules give up energy to do so. The higher the partial pressure the more gas will be dissolved-- thats why your blood boils in a vacuum. Certainly a soft drink usually sealed under pressure will foam more vigorously when it is at room temperature than when it is chilled.
There are several molecular reasons for the change in solubility of gases with increasing temperature which is why there is no one trend independent of gas and solvent for whether gases will become more or less soluble with. This makes the solid particles move faster and farther and thus dissolve in the liquid faster. Gases can exist in significant concentrations within a liquid medium in a variety of chemical forms as discussed below.
Note I say solution and not water. The soda is flat there is little or no carbon dioxide still left in solution. Solubilities of Gases in Water Methane oxygen carbon monoxide nitrogen and helium all have different solubilities in water but all of them become less soluble with increasing temperature.
Theres not enough pressure to keep the gas in it dissolved. Every gas is a finite solubility in every liquid. This shows that gases dissolve best in a cold solution not in a warm solution.
During this process heat is evolved. In general solubility of a gas in water will decrease with increasing temperature. 2011-08-19 12.
Jun 25 2016 at 036. In gases things may dissociate but the mechanism is not about interaction with other things in the gas but because some molecules are unstable and may fall apart into other molecules depending on pressure and temperature. Gases best dissolve in liquids when the liquid temperature is low and when pressure is increased.
The gas molecules in a liquid are dissolved by the process of dissolution. When the pressure of the gas is much larger than the vapour pressure of the solvent the solubility is often proportional to the pressure. Lowering the temperature of a liquid decreases.
More gas can dissolve in cold water than in hot water. According to Le Chateliers Principle which states that when the equilibrium of a system is disturbed the system readjusts itself in such a way that the effect that has caused the change in equilibrium is countered. When the pressure is high and the temperature is high.
And you can demonstrate this every time you take the cap off a bottle of fizzy or carbonated drink. The capacity of gases to exist within liquids is a critical feature allowing for. In fact many gases are more soluble in organic solvents that in water.
This shows that gases dissolves most easily when under pressure. When you wish to dissolve a solid in a liquid faster you increase the temperature. Under which conditions will gases best dissolve in liquids.
When most gasses dissolve into a solution its an exothermic process. When the pressure is low and the temperature is low B. Colder water will be able to have more gas dissolved in it.
The ocean is a ______ because it is a mixture of salts and water. Sugar dissolves in water because the _______ ends of the sugar molecules are pulled off the solute surface by the negative ends of the water molecules. A dissolved gas is simply enough gas that has been dissolved into another material.
OTOH if the temperature of the liquid increases the diffusion of dissolved gas molecules to the interface is faster and the chance of a molecule escaping into the gas phase increases accordingly which is why gases are more soluble in cold water than in warm. A gas is most soluble in water under conditions of high pressure and low temperature. Another common experience is going back to drink a can of coke that has been opened for a while and left in the sun.
See full answer below. This feature of gases can be most directly observed when a can or bottle of soda is opened resulting in release of the carbon dioxide dissolved in the soft drink liquid.

Can Gases Dissolve In Water Chapter 5 The Water Molecule And Dissolving Middle School Chemistry
Mixtures Of Liquids And Gases Henry S Law
13 3 Pressure And Temperature Effects On Solubility Chemistry Libretexts

Can Gases Dissolve In Water Chapter 5 The Water Molecule And Dissolving Middle School Chemistry
No comments for "Under Which Conditions Will Gases Best Dissolve in Liquids"
Post a Comment